Birth Control Implant Lawsuit Help in NJ
Have you or a loved one suffered injury because of a birth control implant or other defective medical device? The New Jersey attorneys at our firm have the knowledge and skill you need now. At Fronzuto Law Group, we concentrate our practice on product liability and medical malpractice litigation, aggressively advocating for injured victims and their families in New Jersey and New York. To discuss your potential claim and find the answers to your questions, contact our New Jersey offices at 973-435-4551 for a cost-free initial consultation.
Failure to Warn about Birth Control Products
Failure to warn is one of the most common types of product liability litigation. The law requires that medical device manufacturers and pharmaceutical companies inform medical professionals of the potential risks associated with their products. If they inform doctors, nurses, and other medical professionals who prescribe or use their products of these risks, medical professionals are then required to inform their patients. In product liability failure to warn cases, it is essential identify where negligence lies. In other words, was it the manufacturer or the medical professional who failed to fulfill their duty? An experienced product liability attorney can assist with determining fault and advocating for those injured by dangerous products.
Wasn’t Informed of the Risk of a Birth Control Implant
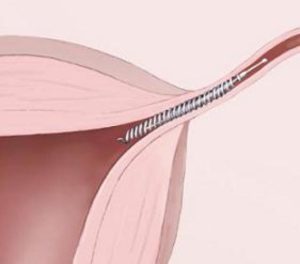
The medical device in this case is the Essure birth control implant, manufactured by Bayer Corp. The underlying argument among the plaintiffs is that Bayer failed to inform medical professionals and the FDA that the implant may pose dangerous health risks, including chronic pain, bleeding, a perforated uterus, and unintended pregnancy.
As for the Food and Drug Administration, this regulatory body enforces the safety standards associated with pharmaceutical drugs and devices. If a manufacturer fails to inform the FDA when findings of potential risks exist, the manufacturer can be held liable for injuries to the innocent patients who utilized their products. In other cases, a manufacturers’ failure to comply with the labeling requirements imposed by the FDA can lead to product liability claims. However, simply providing the appropriate labels does not absolve a medical device or implant manufacturer from liability for failure to warn.
For additional information pertaining to this case, access the following article: In Win for Plaintiffs, Oakland Judge Takes Control of Bayer Suits

